What Is the Best Definition of Specific Heat
The quantity of heat energy that must be absorbed to make one gram of a substance boil 2. However the transfer of energy as heat occurs at the molecular level as a result of a temperature difference.
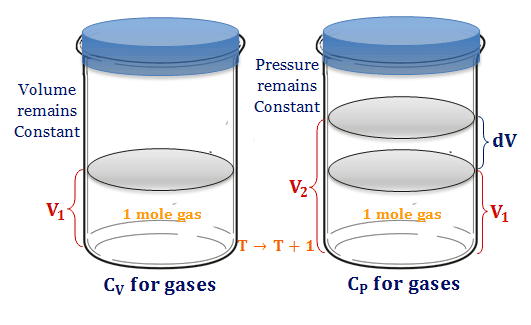
Heat Capacity Gases Definition Calculation Units Formula
How to use heat in a sentence.

. Physiology the sensation caused in the body by heat energy. Heat is not a property of a system. Medical Definition of specific heat.
The meaning of HEAT is to become warm or hot. Water has a specific heat capacity of 4182 JkgC. 717 kJ mol1 5.
The units of specific heat are usually calories or joules per gram per Celsius degree. The ratio of the quantity of heat required to raise the temperature of a body one degree to that required to raise the temperature of an. Specific heat is also known as specific heat capacity or mass specific heat.
Because water is such an important and common substance we even have a special way to identify the amount of energy it takes to raise one gram of water by one degree. The energy transferred as a result of a difference in temperature. 2264 Jg You add 20 J of energy to a sample of water.
For example the specific heat of water is 1 calorie or. It is also an example of an extensive property since its value is proportional to the size of the system being examined. - 66792 kJ 5.
SYMBOL to denote it is c. Heat is a form of energy but it is energy in transit. Similarly heat capacity is the ratio between the energy provided to a substance and the corresponding increase in its temperature.
Why is the specific heat capacity of. Specific heat capacity is the heat needed to raise a substances temperature by 1 degree Celsius. Usually specific heat is reported in joules J.
1 Show answers Another question on Biology. Specific heat is the amount of heat energy required to raise the temperature of a body per unit of mass. The specific heat c is a property of the substance.
The temperature needed to raise the temperature of one gram of a substance by one degree Celsius. The specific heat is the necessary energy to raise the temperature of a mass unit gram kilogram mole of a substance with one kelvin. The definition of specific heat capacity of any substance is the quantity of heat required to change the temperature of a unit mass of the substance by 1 degree This is articulated as.
What is the best definition of specific heat capacity. The molar heat of vaporization for water is 4079 kJmol. It is the lowest level of temperature that can be reached under present ambient conditions brought by water evaporation.
Express this heat of vaporization in Joules per gram. - 33396 kJ 4. The random kinetic energy of the atoms molecules or ions in a substance or body.
The quantity of heat energy that must be absorbed to increase the temperature of onegram of a substance by one degree Celsius correct 2. Use the theory of natural selection to explain how tigers may have evolved to have stripes. The specific heat capacity of a material is a physical property.
The energy in J required to raise the temperature of a substance by 1 K or 1 C. What is the best definition of specific heat. Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass.
Specific heat the quantity of heat required to raise the temperature of one gram of a substance by one Celsius degree. Now best example to Specific heat is Water for water specific heat is 1. The heat needed to raise the temperature of one gram of water by one degree Celsius.
Its SI unit is J kgK or J kgC. The ratio of the amount of heat needed to raise the temperature of a certain amount of a substance by one degree to the amount of heat needed to raise the temperature of the same amount of a reference substance usually water by one degree. As it indicates the resistance of a material to an alteration in its temperature specific heat capacity is a type of thermal inertia.
Specific heat is the amount of heat per unit mass required to raise the temperature by one degree Celsius. Specific heat is measured in this unit per kilogram per degree Celsius. The energy in J required to raise the temperature of 1 kg of a substance by 1 K or 1 C.
What is the best definiton for specific heat. In SI units specific heat symbol. - 437 kJ mol1 correct Explanation.
Water is one of the latterit has a high specific heat capacity because it requires more energy to raise the temperature. Water takes more time to heat up and cool down. The specific heat is the amount of heat necessary to change the temperature of 100 kg of mass by 100ºC.
Calculating thermal energy changes The amount of thermal. The distant ancestors of tigers may have had bodies without stripes. Consider a block of metal at high temperature that consists of atoms that are oscillating intensely around their average positions.
The quantity of heat energy that must be absorbed to increase the temperature of one gram of substance by one degree Celsius. Using both the dry bulb temperature and the wet-bulb temperature we can determine several factors about the air such as relative humidity grains of moisture dew point and enthalpy. The amount of energy of energy it takes to raise the temperature of 1 kilogram of a material 1 celsius degree.
C is the amount of heat in joules required to raise 1 gram of a substance 1 Kelvin. The heat needed to raise the temperature of one gram of water by one degree Fahrenheit. Real life example of specific heat.
Another term used as specific heat. What is the best definition of specific heat. Which of the following is the best definition of specific heat.
Recall that the temperature change ΔT is the same in units of kelvin and degrees Celsius. The quantity of heat energy that must be absorbed to increase the temperature of one gram of a substance. Choose the best definition for this term.
The energy in J required to raise the temperature of 1 g of a substance by 1 K or 1 C. The specific heat capacity of a material is the energy required to raise one kilogram kg of the material by one degree Celsius C.

Specific Heat Definition Facts Britannica

What Is Specific Heat Thermodynamics Chemtalk
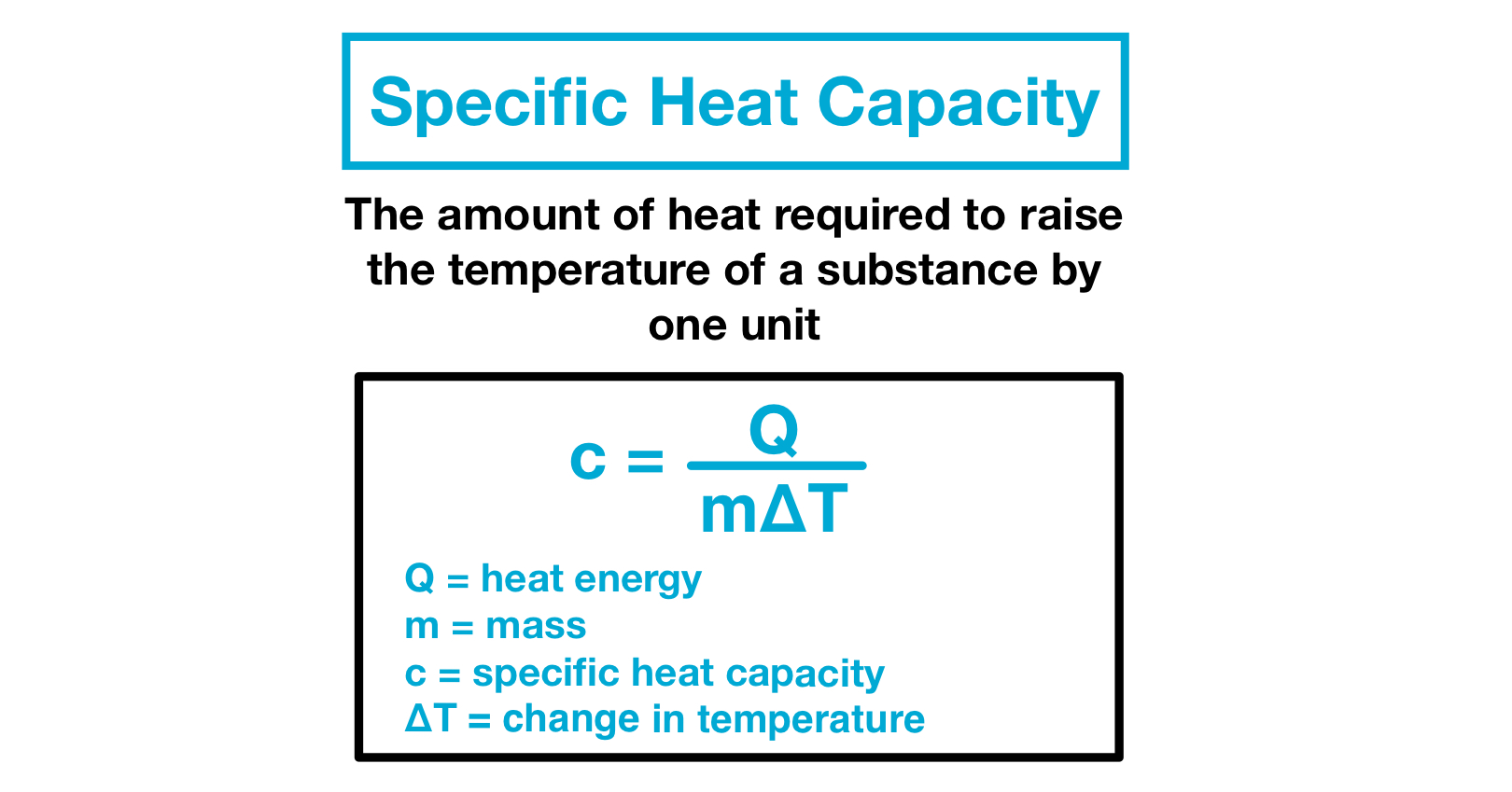
Heat Capacity Of Water Overview Importance Expii
Specific Heat Capacity Matter Physics Fuseschool Youtube
Specific Heat Heat Of Vaporization And Density Of Water Article Khan Academy

Specific Heat Boundless Physics
Difference Between Cv And Cp Definition Properties Formula
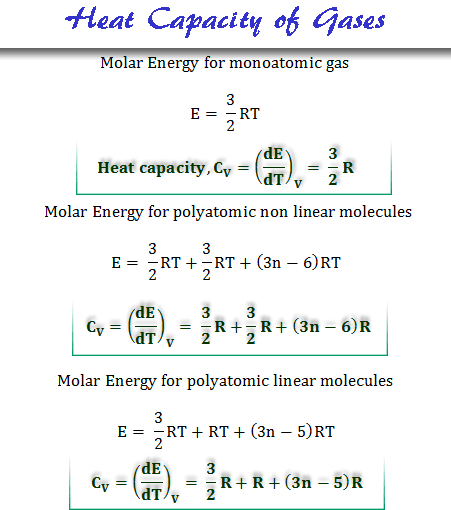
Heat Capacity Gases Definition Calculation Units Formula

Specific Heat Capacity Definition Molar Specific Heat Videos Examples

Specific Heat Capacity Definition Molar Specific Heat Videos Examples
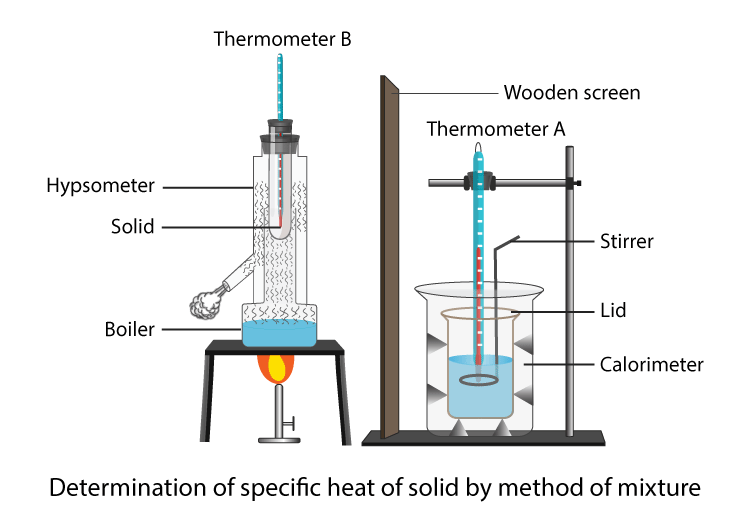
To Determine Specific Heat Capacity Of A Given Solid Physics Practical

Specific Heat Boundless Physics

Selina Solutions Concise Physics Class 10 Chapter 11 Calorimetry Avail Free Pdf

Specific Heat Easy Science Thermal Energy Chemical Energy Physics Concepts

What Is Heat Capacity Definition Equation With Videos
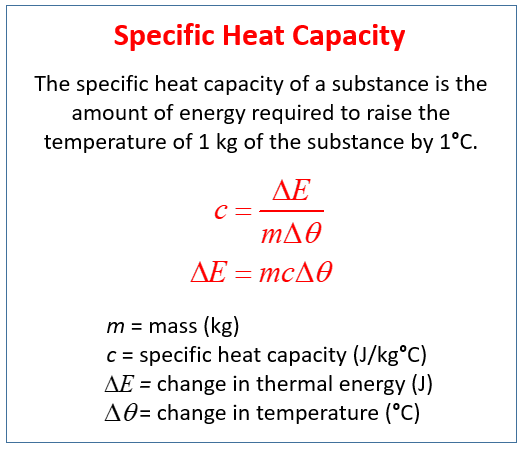
Specific Heat Capacity Video Lessons Examples Step By Step Solutions

Specific Heat Capacity Water Formula Detailed Explanation With Videos Examples

Difference Between Specific Heat And Heat Capacity With Table Ask Any Difference

Specific Heat Capacity Definition Molar Specific Heat Videos Examples
Comments
Post a Comment